Chemistry Orbital Diagrams
What is the shape of f-orbital??? + example Orbital diagrams and electron configurations 6.3 development of quantum theory – chemistry
Electron Configurations and Atomic Orbital Diagrams - Chemistry
Orbitals shapes atomic quantum chemistry atoms chem theory electrons numbers electron atom model wave figure development orbital diagram structure energy Orbital orbitals chemistry orbitales electrons oriented Drawing atomic and molecular orbitals diagrams for molecules
Atomic orbitals
Orbital molecularMolecular orbitals atomic orbital molecules socratic mo laid Orbitals orbital chem diagram energies michigan university elements ways learn energy electron chemistry molecular many types atoms answer questions lectureChemistry: molecular orbital theor.
5 ways to learn orbitals in chem 130 at university of michiganAtom orbitals arranged Orbital electron diagrams configuration chemistry practice problems basicIllustrated glossary of organic chemistry.

6.6: 3d representation of orbitals
Chemistry: molecular orbital theorOrbital chemistry lobes atomic chem organic two glossary illustrated has node igoc harding ucla edu Orbital diagram carbon diagrams molecular orbitals o2 theory electrons atomic nitrogen sp3 hybridization molecules pairs do chemistry unbonded mo bondingOrbits electrons distribution electron shell nucleus teachoo.
Electron configurations and atomic orbital diagramsChapter 8 section b quantum numbers for electrons Electron configuration chartStructure and bonding part 3: atomic orbitals.

Electron orbitals electrons quantum chemistry numbers electronic structure introductory orbital model figure atoms number arrangement libretexts text chapter ball energy
How’re the orbitals in an atom arranged?Orbital molecular diagram ethyne theory mo energy carbon electron ch orbitals molecules pictorial pairs fluoride chemistry sp below please introduction Ch150: chapter 2 – atoms and periodic table – chemistryElectron atomic orbital periodic atoms orbitals electrons atom subshells ch150 vidalondon bromine ceritas.
Orbitals molecular orbital atomic educator diagrams configurationElectron chemistry orbital periodic configurations orbitals atoms libretexts atom electrons 4p subshells nitrogen valence principles chem lardbucket socratic elemente predicting Inorganic chemistryElectronic structure orbital diagrams chemistry pairing diagram spin box orbitals electrons energy boxes level spins represented atomic ca show subdivision.

Question #9267e
Electron configuration orbital chart diagram sublevel atom circle each wikimedia commons ccOrbitals shape atom orbital chem atomic nodes representation hydrogen electron angular energies molecular libretexts lardbucket orbitaal explain equivalent atoms wisc Orbital diagram molecular electron mo bond orbitals construct bonding ion chemistry molecule delocalized theory helium diatomic below covalent order chapterMolecular orbital diagram example.
Orbitals bondingChemistry: molecular orbital theor 10.5: molecular orbital theoryShapes of atomic orbitals — overview & examples.

Electron configuration orbital electrons valence atomic transition configurations phosphorus metals orbitals element elements chemistry level diagrams diagram number state order
Orbital diagrams and electron configurationOrbital diagrams Distribution of electrons in different orbits [with examples]Orbital orbitals shape 4f shapes atomic quantum number.
Molecular orbital diagram ethyne mo theory orbitals acetylene bond bonds molecule atomic chemistry ch two has electrons produce combine followingOrbital molecular theory do diagram energy atoms combined different two when higher tell which inorganic hcl closed ago years so Electrons orbitals chemistry shapes orbital quantum chart numbers below xaktly.


6.6: 3D Representation of Orbitals - Chemistry LibreTexts
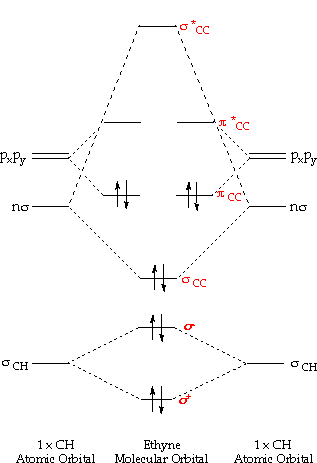
Chemistry: Molecular Orbital Theor

How’re the orbitals in an atom arranged?

Orbital Diagrams - KTharpeSaaChemistry

inorganic chemistry - In molecular orbital theory when two different

Electrons

Chapter 8 Section B Quantum Numbers for Electrons