Bonding And Antibonding Orbitals Order
Atomic physics Bonding molecular antibonding orbital orbitals between mo bonds theory difference pi diagram covalent multiple ethylene energy electron level chemistry polyatomic 12 difference between bonding and antibonding molecular orbitals
what is anti bonding and bonding orbitals - Chemistry - Chemical
Orbitals bonding anti phase overlap equation schrödinger pi molecular chemistry non dependent light time schrodinger blue diagram depending whether stack Localized bonding and hybrid atomic orbitals Condensed matter
9.6: multiple bonds
Diagram orbital molecular ozone bonding orbitals molecule antibonding nonbonding bonds delocalized electrons resonance chemistry multiple polyatomic non benzene example moBonding anti orbitals Supplementary illustrationsOrbitals overlap atomic chemistry orbital bonding covalent hybridization localized px pz hybrid py bond molecular electron form different figure general.
Bonding orbital orbitals theories valence antibondingHow to calculate bond order from molecular orbital diagram Molecular orbital diagram diatomic molecules cl2 theory orbitals bonding bond delocalized second row electron diagrams energy h2 chemistry level homonuclearIllustrated glossary of organic chemistry.

Bonding antibonding orbitals apbx vb
9.5: bonding and antibonding orbitalsExplain the terms (i) bonding molecular orbitals(ii) antibonding Antibonding orbitals orbital first bonding molecular mo 2s 2p electrons mos diatomic theory species energy filled why 1s diagram atomicChapter 8 presentation.
Bonding antibonding 1s mo orbitals two form mos combination atoms atomic mo2 hydrogen relative stabilities does called between shaunmwilliams genchemSolved indicate on the diagram the bonding, antibonding Orbital molecular orbitals antibonding bondingBonding orbitals molecular orbital antibonding non theory according many homeworklib benzene re there ti os foto energy too.

Bonding covalent actually does work molecule physics
Bonding molecular orbitals antibonding orbitalAntibonding orbital orbitals chemistry molecular phase overlap illustrated glossary organic component bond forms Antibonding orbital bonding molecular difference orbitalsBonding antibonding orbitals bonded electrons atoms bond together but ppt powerpoint presentation energy.
Quantifying individual (anti)bonding molecular orbitals’ contributions3. according to molecular orbital theory, how many re-bonding molecular Why are antibonding orbitals filled first? + exampleOrbital molecular diagram orbitals antibonding bonding chemistry libretexts energy theory use stable level delocalized h2 1s bond atomic electrons which.

What is anti bonding and bonding orbitals
Bonding nonbonding antibonding solved orbital diagram indicate transcribed problem text been show has hydrogenPolyatomic systems with multiple bonds Orbitals bonding antibonding molecular presentation between mo energy(a) a simplified molecular orbital diagram shows the bonding and.
Molecular orbitals bond many orbital nitrogen theory molecule electrons diagrams diagram antibonding bonding correlation fluorine atom valence description supplementary clickingA) schematic representation of bonding/antibonding orbitals of apbx 3 Bonding theories valence bond theory molecular orbital theoryDelocalized bonding and molecular orbitals.

Orbital molecular pi antibonding bonding orbitals electron electrons construct go
Antibonding bonding orbitals molecular vs between differencePhysical chemistry Orbitals bonding contributions quantifying individualWhat is anti bonding and bonding orbitals.
Orbital bonding simplifiedBonding orbitals antibonding energy bands physics state band anti condensed matter there Bonding orbitals anti molecularDifference between bonding and antibonding molecular orbital.


atomic physics - How does covalent bonding actually work? - Physics

Polyatomic Systems with Multiple Bonds
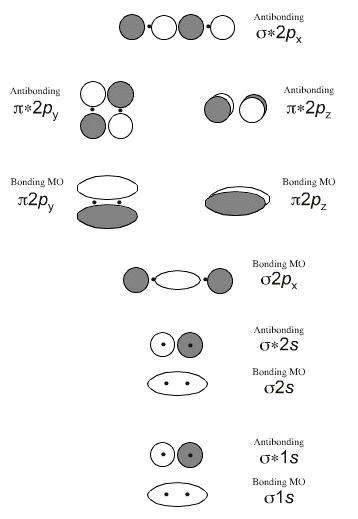
Why are antibonding orbitals filled first? + Example

what is anti bonding and bonding orbitals - Chemistry - Chemical

9.6: Multiple Bonds - Chemistry LibreTexts

PPT - Atoms are bonded together by electrons , but what is a bond

Solved Indicate on the diagram the bonding, antibonding | Chegg.com